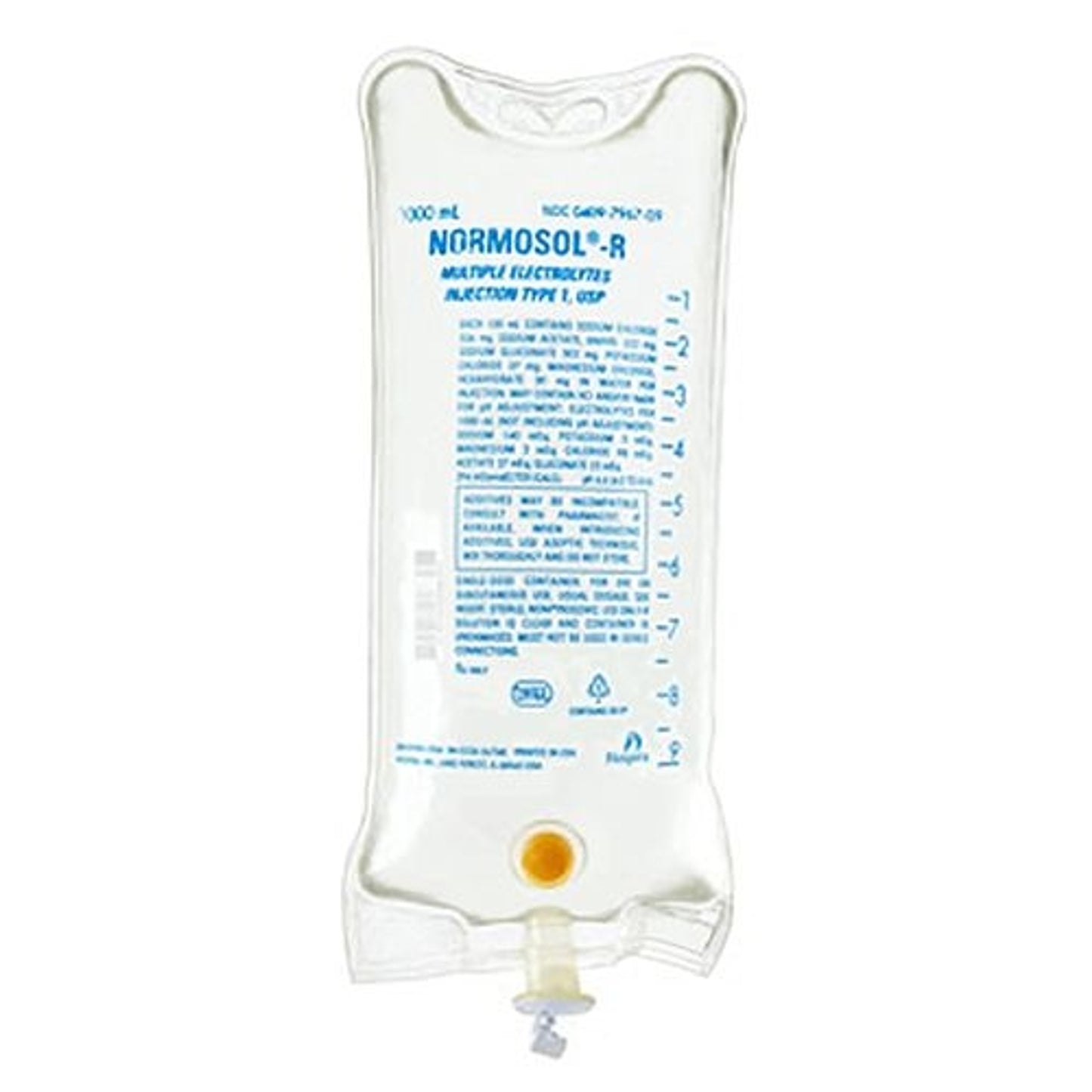
Normosol
Normosol R 1 liter
Normosol R 1 liter
Couldn't load pickup availability
Prescription items are NON-RETURNABLE and NON REFUNDABLE
NORMOSOL®-R 1 liter
SINGLE DOSE.
Contains no preservative.
Electrolytes per 1000 mL (not including pH adjustment): Sodium,140 mEq; potassium,5 mEq; magnesium, 3 mEq; chloride, 98 mEq; acetate, 27 mEq; gluconate, 23 mEq.
IMPORTANT: CONTAINS NO PRESERVATIVE. SINGLE DOSE. DISPOSE OF UNUSED CONTENTS.
Protect from freezing and excessive heat (any temperature above 40°C / 104°F). Store at controlled room temperature (15°C to 30°C / 59°F to 86°F).
CONTRAINDICATIONS
None known.
WARNINGS
Solutions containing sodium ions should be used with great care,if at all in patients with congestive heart failure,severe renal insufficiency and in clinical states in which there exists edema with sodium retention.
Solutions which contain potassium should be used with great care if at all in patients with hyperkalemia severe renal failure and in conditions in which potassium retention is present.
In patients with diminished renal function administration of solutions containing sodium or potassium ions may result in sodium or potassium retention.
Solutions containing acetate or gluconate ions should be used with great care in patients with metabolic or respiratory alkalosis. Acetate or gluconate should be administered with great care in those conditions in which there is an increased level or an impaired utilization of these ions such as severe hepatic insufficiency.
The intravenous administration of this solution can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations overhydration congested states or pulmonary edema.
The risk of dilutional states is inversely proportional to the electrolyte concentrations of administered parenteral solutions. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentrations of such solutions.
RECAUTIONS
NORMOSOL®-R should be used with caution in severe renal impairment because of the danger of hyperkalemia. As with all intravenous solutions care should be taken to avoid circulatory overload especially in patients with cardiac or pulmonary disorders. NORMOSOL®-R is not intended to correct acidosis or large deficits of individual electrolytes nor to replace blood or plasma expanders when these are indicated.
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance electrolyte concentrations and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Caution must be exercised in the administration of parenteral fluids especially those containing sodium ions to patients receiving corticosteroids or corticotropin.
Solutions containing acetate or gluconate ions should be used with caution as excess administration may result in metabolic alkalosis.
Do not administer unless solution is clear and container is undamaged. Discard unused portion.
ADVERSE REACTIONS
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection,venous thrombosis, or phlebitis extending from the site of injection,extravasation and hypervolemia.
If an adverse reaction does occur discontinue the infusion evaluate the patient institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
OVERDOSAGE
In the event of overhydration or solute overload re-evaluate the patient and institute appropriate corrective measures. See WARNINGS PRECAUTIONS and ADVERSE REACTIONS.
NORMOSOL®-R is administered by intravenous infusion. It may also be administered subcutaneously. The amount to be infused is based on replacement of losses of extracellular fluid volume in the individual patient. Up to 3 times the volume of estimated blood loss during and after surgery can be given to correct circulatory volume when there is only a moderate loss of blood.
Drug Interactions
Additives may be incompatible. Consult with Additive Manufacturer. When introducing additives use aseptic technique mix thoroughly and do not store.
Parenteral drug products should be inspected visually for particulate matter or discoloration prior to administration whenever solution and container permit.
NORMOSOL®-R does not contain calcium to avoid precipitation of calcium salts that may occur when certain drugs are added. Solutions which contain calcium in amounts exceeding the normal plasma concentration may enhance clotting on contact with citrated blood. Hence,NORMOSOL®-R can be used for starting blood transfusion.
INSTRUCTIONS FOR USE
To Open
Tear outer wrap at notch and remove solution container. If supplemental medication is desired follow directions below before preparing for administration. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
To Add Medication
- Prepare additive port.
- Using aseptic technique and an additive delivery needle of appropriate length puncture resealable additive port at target area inner diaphragm and inject. Withdraw needle after injecting medication.
- The additive port may be protected by covering with an additive cap.
- Mix container contents thoroughly.
Preparation for Administration (Use aseptic technique)
- Close flow control clamp of administration set.
- Remove cover from outlet port at bottom of container.
- Insert piercing pin of administration set into port with a twisting motion until the set is firmly seated. NOTE: See full directions on administration set carton.
- Suspend container from hanger.
- Squeeze and release drip chamber to establish proper fluid level in chamber.
- Open flow control clamp and clear air from set. Close clamp.
- Attach set to venipuncture device. If device is not indwelling prime and make venipuncture.
- Regulate rate of administration with flow control clamp.
Prescription items are NON-RETURNABLE and NON-REFUNDABLE.
SKU:E024052
Model Numbers and UPCs
Model Numbers and UPCs
| Style | Item Number | UPC |
|---|---|---|
| One Size | E024052 |
Prop 65 Warning
Prop 65 Warning
⚠️ WARNING: This product can expose you to chemicals which is known to the State of California to cause cancer, birth defects or other reproductive harm. For more information, go to www.P65Warnings.ca.gov
Share